Corrosion of reinforcement in HVFA concrete - I

Corrosion of reinforcements has been one of the foremost challenges the civil engineers have been facing these days. Corrosion leads to the creation of rust which results in the spalling of concrete. This, in turn, leads to the exposure of rebars to the aggressive environment. This will speed up the ill effects and eventually leads to the breakdown of the structure. However, corrosion mainly occurs in areas of the aggressive environment such as coastal regions. The most important thing is to prevent the corrosion of reinforcement in order to have a durable arrangement. Even though there are many methods to prevent the corrosion, most of them are uneconomical and require great skill. While the recent studies in various parts of the world have revealed that High Volume Fly Ash (HVFA) concrete can protect the steel strengthening more efficiently so that it can resist corrosion, and therefore the structure as a whole. HVFA concrete is a type of concrete in which a part of the cement is replaced by fly ash, which is again an industrial waste. Thus the implementation of H V F A concrete can minimize corrosion in an effective way. Moreover, it can lead to a much strong structure without a considerable increase in cost.
While the use of concrete containing HVFA has recently gained popularity as a resource which is efficient, durable, and sustainable for a variety of concrete applications. The two HVFA mixtures - one containing Class C fly ash the other Class F fly ash, were compared with TDOT Class A common use mixtures using the similar class of fly ash at a lesser replacement percentage.
However, the HVFA mixtures reached similar to higher long-term compressive strengths, due to the pozzolanic properties of the fly ash and the lower w/cm ratios. Also, the water permeable void contents and absorptions were lower for the HVFA mixtures at all ages, this indicates that the durability of the HVFA is much better than that of the TDOT mixtures. The setting times for the HVFA mixtures were about two hours longer than those of the TDOT Class A mixtures at laboratory conditions (72oF (22oC)). Also, the costs of the HVFA mixtures were slightly advanced. Overall, the use of HVFA mixtures is an ideal idea for warm weather placements; when compared with the TDOT Class A mixtures, the HVFA mixtures display comparable costs, increased compressive strengths, and enhanced durability properties.
Let us see the factors that influence the ability of the reinforcing steel to remain passivated are the water to cement ratio, permeability and electrical resistance of concrete. For the improvement of a service life of reinforced concrete structures exposed to corrosive environments, the use of corrosion-inhibiting admixtures, epoxy-coated reinforced steel, and cathodic guard are among the better known technological advancements.
The literature on reinforcement corrosion in concrete structures, its mechanism, and factors affecting corrosion of steel in concrete are accessible. In this blog we will see about the various aspects of the reinforcement corrosion, the service life of the structure and role of inhibitors as listed have been discussed:
- Factors affecting reinforcement corrosion
- Service life forecast of corroded structure
- Role of inhibitors on service life of reinforced concrete
Factors affecting reinforcement corrosion
Corrosion of reinforcement in the concrete structure is an electrochemical procedure and is similar to the action which takes place in a flash battery. The ‘anodic reaction’ which is the oxidation process and is reliant on the pH of interstitial electrolyte, presence of aggressive anions and the continuation of an appropriate electrochemical potential at the reinforcing bar surface, results in termination or loss of metal whilst the ‘cathodic reaction’ which is a decrease process and is dependent on the availability of O2 and the pH in the vicinity of re-bar. These anodic and cathodic reactions are broadly referred to as ‘half-cell reactions’ and the equivalent electrode potential can be derived by Nernst equation. The electromotive force (e) of reinforcement corrosion cell which is the difference between EC and EA drives the corrosion current through the electrolyte from anode and cathode.
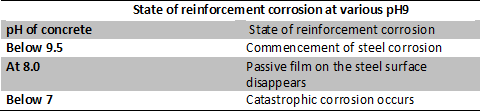
Effect of Carbonation and Entry of Gaseous Pollutants
The pH of the concrete is condensed by the carbonation and entry of acidic gaseous pollutants such as SO2 and NO2. However, the fall in pH to certain levels can cause commencement of reinforcement of corrosion and loss of passivity of concrete against rebar corrosion along with catastrophic corrosion. The same is explained in the below table.
Effect of Destructive Anions
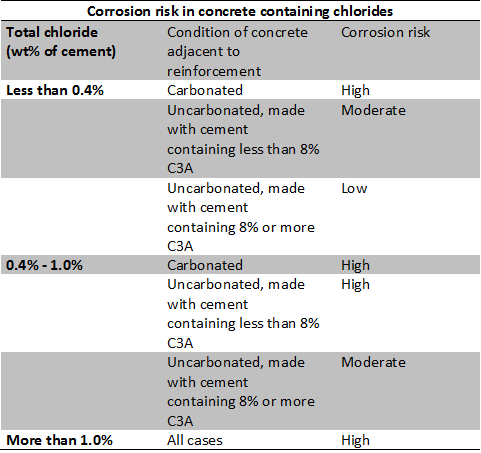
Chloride in concrete may be present as Acid soluble chloride, chemically bound chloride with hydration products of cement and free or water soluble chloride within the pore solution of concrete. Normally, the concentration of free chloride ions (Cl-) influences the corrosion process. It is reported that the corrosion rate increases with an increase in chloride content. However, the change in pH remains insignificant due to change in chloride content of concrete. While the risk of reinforcement corrosion is associated with the level of chloride content in both uncarbonated and carbonated concrete. See the below table to get the detailed description.
Effect of Bacterial Action
Aerobic bacteria may help in the formation of different aeration cell which will guide to corrosion. While in sewer concrete, the anaerobic bacteria produces iron sulfides which too enables the corrosion response to proceed even in absence of oxygen9. however, the bacteria decreases the amount of cover by the disintegration of cementitious material.
Effect of w/c ratio
Essentially w/c ratio control strength, durability, and permeability of concrete do not control the rate of corrosion but ‘permeability’ which is a function of w/c ratio affects the corrosion of rebar. The penetration depth of a particular chloride threshold value increases with an increase in the w/c ratio. You can see the carbonation depth to be linearly increasing with an increase in w/c ratio. While the oxygen diffusion coefficient is also found increasing with an increase in the w/c ratio. This is observed in a recent study that the permeability of hardened cement paste is increased 100 fold by increasing the w/c ration from 0.35 to 0.45 and the time of initiation of support corrosion in a sample with a w/c ration 0.4 is 2.15 to 1.77 times more as compared to a sample with a w/c ratio of 0.55, under accelerated corrosion testing.
Effect of Cover over Reinforcing Steel
The risk of reinforcement corrosion with low cover thickness has been discovered by various researchers, and the cover thickness has a remarkable effect on rebar corrosion due to penetration of chloride or carbonation. However, this effect of corrosion is limited within the time of casting to the time at which the rebar is de-passivated and corrosion is in progress. The rate of corrosion once started will be independent in regards to the cover thickness.
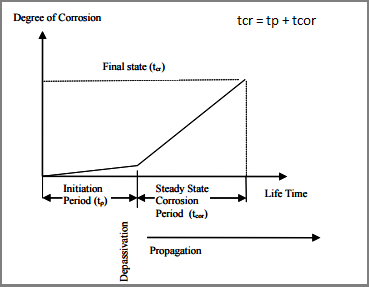
Service life prediction of structure
The state of corrosion of steel in concrete is a function of time. Corrosion process of Reinforcement in concrete starts with de-passivation which is a loss of oxide layer over the rebar and then it propagate to reach a significant stage at which corrosion would produce spalling of concrete cover or cracking through the whole concrete as depicted. Hence the service life of concrete may be expressed in the below image:
This is a part of the blog on Corrosion of reinforcement in HVFA concrete, in coming blog we will see about the stages of rebar corrosion and the Role of inhibitor on service life of reinforced concrete.