LEDs from Food and Beverage waste
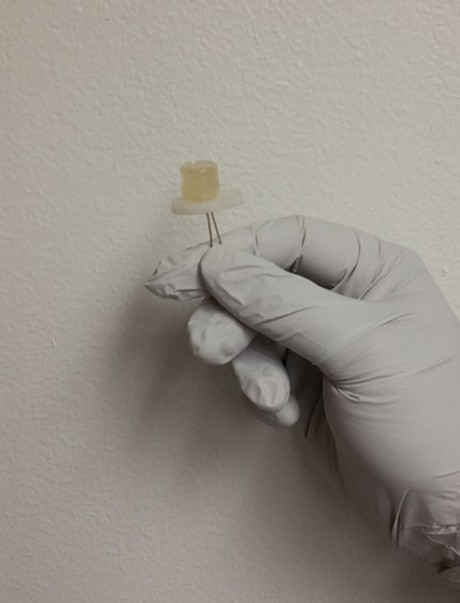
LEDs – Light Emitting Diodes have been an efficient alternative to incandescent and fluorescent bulbs for several years. The researchers from the University of Utah found a way to discover LEDs even more sustainable than they already are, and in this innovation they have used waste food and beverage.
These LEDs are created by using quantum dots (QDs), or small crystals that have luminescent properties, to produce light. Quantum dots are made of numerous materials and some of the materials are rare and expensive to synthesize and even potentially injurious as they dispose of.
Since last decade research has been on the move, and focused on using carbon dots or quantum dots to create LEDs. However, carbon dots have low toxicity and enhanced biocompatibility, which makes them suitable for a wide range of applications.

Metallurgical engineering and research assistant at University of Utah, Prashant Sarswat, and Professor Michael Free worked more than a year and a half to manage and turn the discarded pieces of tortilla into carbon dots and, then subsequently, LEDs.
Free and Sarswat employed a solvothermal synthesis, in which the waste food was placed into a solvent under high temperature and force until CDs were formed. In this experiment both the researchers used bread and tortilla pieces along with soft drinks. These components were placed in a solvent then heated for 30 to 90 minutes. After finding traces of carbon dots, the researchers proceeded to light up the dots to examine their color and formation, the researchers also tested through four other tests - Fourier transforms infrared spectroscopy, Raman and AFM imaging, x-ray photoelectron spectroscopy to determine the CDs’ optical and material properties.
Synthesizing and characterizing carbon dots derived from waste is a very exigent task, said Sarswat. we essentially have to verify the size of dots which are only 20 nanometers or smaller in diameter, that is the reason we need to perform multiple tests to be sure that the carbon dots are present and to find out what optical properties they own.
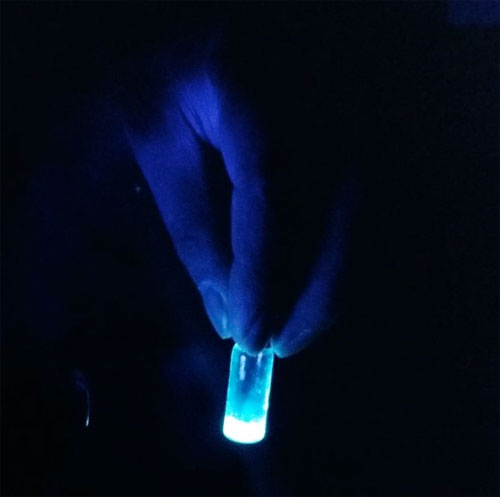
The various tests helped us to measure the size of the carbon dots, which correlates with the intensity of the dots’ brightness & color. Then we have to determine which carbon source produces the best CDs. For example, if D-fructose and sucrose dissolved in soft drinks were found as effective sources for carbon dot production, then finally these CDs were suspended in epoxy resins, heated to harden for use in LEDs. Moreover breaking down Cadmium selenide is an expensive process and creating QDs from waste food and beverage will potentially eliminate these toxicity concerns.
Going forward, both the researchers hope to continue studying the LEDs produced from food and beverage waste will for better stability and long-term performance. The ultimate objective is to do this on a mass range and to utilize these LEDs in everyday devices and effectively make use of waste that already exists, that’s the end goal.





